For a given Period, which is the smallest atom?
1 Answer
Jul 19, 2017
The inert gases are in fact the SMALLEST atoms of their respective Periods.
Explanation:
Do these data support the opening statement?
Units of
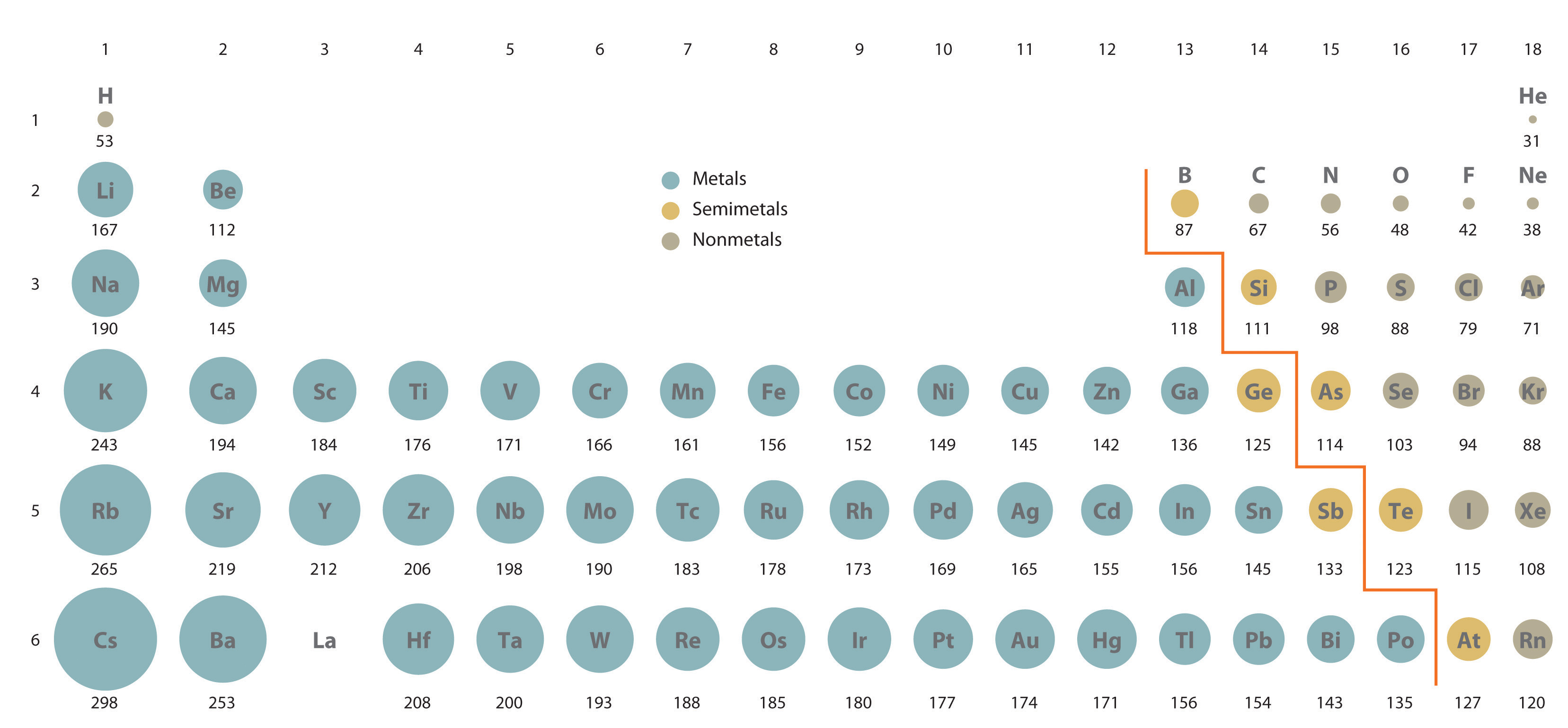
And as we face the Table, atomic size DECREASES across the Period from right to left, and INCREASES down a Group, a column of the Periodic Table.
The Periodic decrease is a consequence of electronic structure. Incomplete electron shells shield the nuclear charge very ineffectively. Across the Period,

