How can the octet rule be violated?
1 Answer
The octet rule is violated whenever a bonded atom has either fewer or more than eight valence electrons in its valence shell.
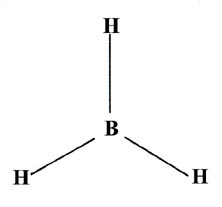
INCOMPLETE OCTET
BH₃ has only six valence electrons around B. The B atom has an incomplete octet.
ODD-ELECTRON MOLECULES
Nitrogen monoxide, NO, has 11 valence electrons. There is no way that both atoms can get an octet. One atom is always stuck with only 7 electrons in its valence shell.

FREE RADICALS
Free radicals are reactive intermediates that are formed during reactions, for example, by removing an H atom with its shared electron from a molecule such as CH₄. They have incomplete octets and unpaired electrons.

EXPANDED OCTETS
The nonmetals after silicon in the Periodic Table can “expand their octet” and have more than eight valence electrons around the central atom. An example is PF₅, with 10 electrons in its valence shell.


