How do metals obey the octet rule?
1 Answer
Only the metals in Groups 1 and 2 strictly obey the octet rule.
Explanation:
Group 1 and Group 2 Metals
Metals in Group 1 can lose one
Transition Metals (Group 3 to Group 11) and Group 12
Transition metals usually have an electron configuration that ends in
They often lose two or three of their valence electrons, but not enough to get back to an
For example, the electron configuration of
Transition metals will often violate the octet rule by using their
They can expand their octet to twelve or more valence electrons.
An example is hexaamminecobalt(III) chloride.
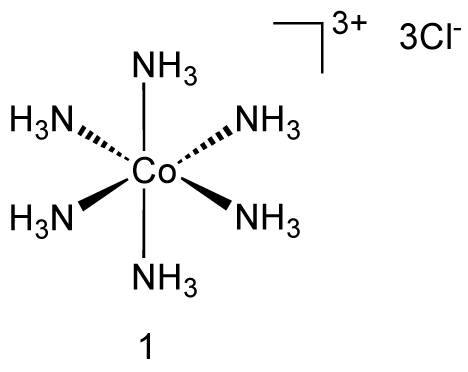
Groups 13 to Group 15 Metals
Aluminium forms compounds such as
All the other metals in Groups 12 to 15 can expand their octets.

