How do pi and sigma bonds relate to hybridization?
1 Answer
Most atoms hybridize their s and p atomic orbitals to get the most stable molecular orbitals.
Explanation:
An atom's valence shell has one s orbital and three p orbitals.
When two s orbitals overlap, they form a σ bond.
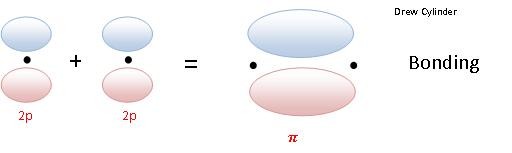
When two p orbitals overlap side-to-side, they form a π bond.
s and p orbitals can form different hybridizations.
s + p → sp
s + p + p → sp²
s + p + p + p → sp³
When two hybridized orbitals overlap, they form a σ bond.
sp³-hybridized atoms use all three p orbitals for the hybridization. This means that sp³ hybridized atoms can form only sigma bonds. They cannot form multiple bonds.
sp²-hybridized atoms use only two p orbitals in the hybridization. There is an unused p orbital. This left-over p orbital can create a π bond with another atom and create a double bond.

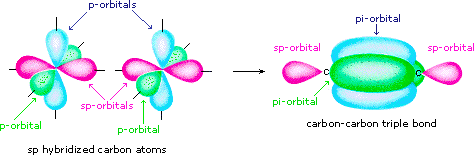
sp-hybridized atoms use only one p orbital in the hybridization. This means you can create two π bonds.