What is the orbital hybridization in BrCl3?
1 Answer
In bromine trichloride, or
Start by drawing the Lewis structure of the compound. The total number of valence electrons will be 28, 7 from each of the three chlorine atoms and 7 from the bromine atom.
 )
)
Out of the 28 valence electrons, 24 will be used to complete the octets of the chlorine atoms - each chlorine atom has 3 lone pairs and shares single bond with bromine.
As you can see, the remaining 4 valence electrons are placed on bromine as lone pairs. This means that bromine will have a total of 10 valence electrons, which is possible because it can have an expanded octet.
The hybridization of the bromine atom is determine by counting the regions of electron density that surround the atom - this represents the steric number.
So, bromine has 2 lone pairs, and 3 single bonds, which means it has a steric number of 5. The steric number will also tell you how many hybrid orbitals an atom has. In this case, 5 hybrid orbitals would correspond to an
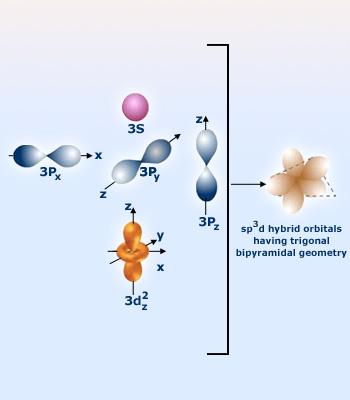
Because of the two lone pairs of electrons, the molecular geometry of the bromine trichloride molecule will be T-shaped, not trigonal bipyramidal.
 )
)


