What are the different types of atomic orbitals?
1 Answer
There are many types of atomic orbital (
Explanation:
Quantum numbers
Two quantum numbers determine the type of orbital.
The principal quantum number,
The secondary quantum number,
For each value of
These orbitals are spheres.
The higher the value of
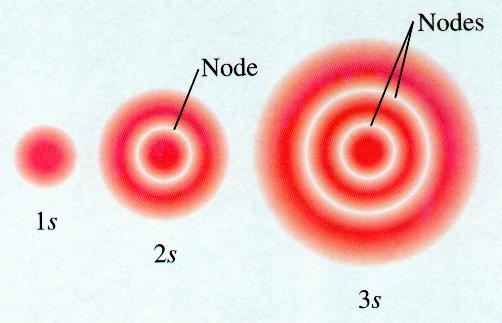
The spheres like nested shells separated by nodes — areas where there is no electron density.
When
When
A
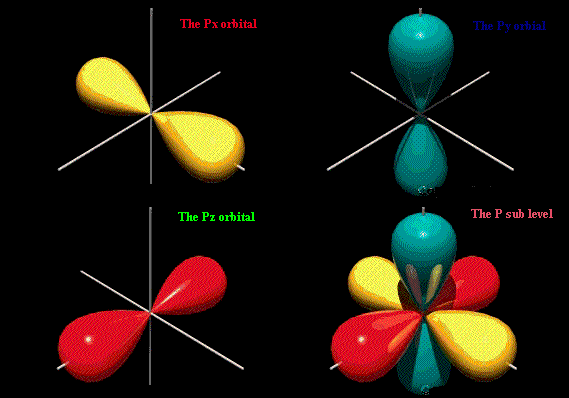
There are three types of
Each points in a different direction.
When
These are called

One looks like a dumbbell with a doughnut around the middle.
The other four
When
These are called
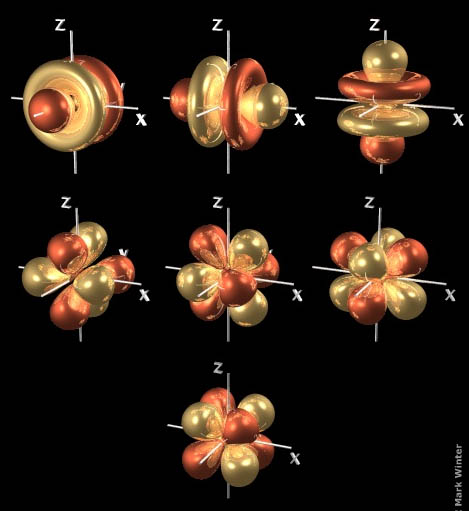
Three of the orbitals look like a dumbbell with two donuts around the middle.
The other four orbitals look like a bundle of eight balloons tied together and pointing to the corners of a cube.

