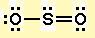How to draw chemical bonding?
1 Answer
It depends whether the bonds are ionic or covalent.
Explanation:
IONIC BONDS
Ionic bonds are formed when a metal transfers an electron to a nonmetal, so that each atom completes its octet of valence electrons.
For example, sodium has one valence electron, while chlorine has seven valence electrons.
If a sodium atom donates an electron to a chlorine atom, both atoms will have an octet of valence electrons.
Since the
The
The ionic bond consists simply of the electrostatic attraction between the positive and negative ions.
We write the valence electrons as dots. We then enclose the ions in brackets with the charges written as superscripts and write the ions side by side.
Thus, the structure of

COVALENT BONDS
Atoms can also form a stable octet around each atom by sharing electrons.
A bond is the sharing of two electrons.
We usually represent a bond as a dash that represents two electrons.
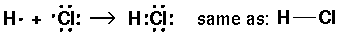
If the molecule has more than two atoms, we usually have to draw a Lewis structure.
Example
How do I draw the bonds in
Solution
- Decide which atom is the central atom in the structure. That will normally be the least electronegative atom (
#"S"# ). - Draw a skeleton structure in which the other atoms are single-bonded to the central atom:
#"O-S-O"# . - Draw a trial structure by putting electron pairs around every atom until each gets an octet. In this editor, I will have to write it as
#"::Ö-S(::)-Ö::"# - Count the valence electrons in your trial structure (20).
- Now, count the valence electrons you actually have available.
#1 "S" + 2 "O" = 1×6 + 2×6 = 18# . The trial structure has two electrons too many. - Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:
#"O-S=O"# . - As before, add valence electrons to give each atom an octet:
Note that shared pairs of electrons are drawn as dashes, but unshared electrons are shown as pairs of dots.