What are the exceptions to octet rule with odd number electrons?
1 Answer
Feb 14, 2014
Molecules with an odd number of electrons are exceptions to the octet rule because there is no way for all atoms to achieve an even-numbered octet.
Consider nitrogen monoxide, NO. It has 11 valence electrons. There is no way that both atoms can get an octet.

NO₂ and ClO₂ are other molecules with odd numbers of valence electrons

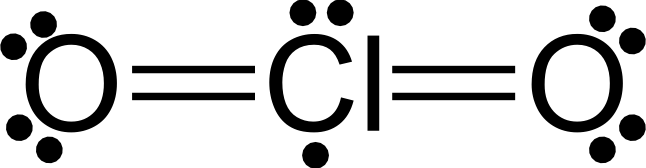
FREE RADICALS
Free radicals such as CH₃· and HO· are odd-electron species that formed during reactions by removing an atom such as H with its shared electron from stable molecules such as CH₄ and H₂O.



