Why do solutes lower vapor pressure?
1 Answer
Solutes lower vapour pressure because they get in the way of solute particles that might escape into the vapour.
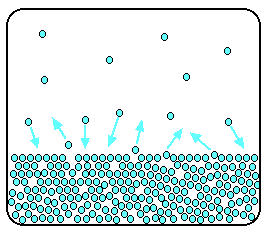
In a sealed container, an equilibrium is set up in which particles leave the surface at the same rate as they return.
Now suppose you add enough solute so that the solvent molecules occupy only 50% of the surface.
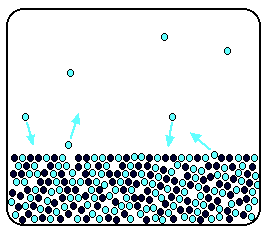
Some of the solvent molecules still have enough energy to escape from the surface. If you reduce the number of solvent molecules on the surface, you reduce the number that can escape in any given time.
It makes no difference to the ability of molecules in the vapour to stick to the surface again. If a solvent molecule in the vapour hits a bit of surface occupied by the solute particles, it may well stick.
The net effect is that when equilibrium occurs, there are fewer solvent molecules in the vapour phase. It is less likely that they are going to break away, but there is no problem about their returning.
If there are fewer particles in the vapour at equilibrium, the saturated vapour pressure is lower.

