Why does Beryllium form an sp hybrid orbital?
I noticed that when #Be# reacts with #H_2# it forms an sp hybrid orbital even though the #Be# electron configuration is #1s^2 2s^2# and thus the electrons do not occupy a #p# orbital. Am I wrong in saying that an element's electrons need to occupy an orbit to it to exhibit a hybrid orbital with that respective orbital?
I noticed that when
1 Answer
In this it has nothing to do with bond angles not being
The issue here is the orbital phases are incorrect for a bonding molecular orbital.
- The
#2s# orbital does not stick out far enough to bond with two atoms at the same time. - The
#2p# orbital is the opposite phase on one side, which would have meant making two DIFFERENT#"Be"-"H"# bonds.
Upon hybridization, two IDENTICAL bonds can be made, to give:
 )
)
instead of:
 )
)
I assume you are referring to the formation reaction:
#"Be"(s) + "H"_2(g) -> "BeH"_2(g)# ,#DeltaH_f^@ = "125.52 kJ/mol"#
It does not matter that the
Orbital hybridization is a theory invented by Linus Pauling, and we only use this theory to help describe known molecular geometries around the CENTRAL atom only, that
#(i)# utilize angles that are not#90^@# and/or
#(ii)# form multiple identical bonds even though different instead of identical pure orbitals are available.
In this theory for beryllium, we know that
#" "" "" "underbrace(ul(color(white)(uarr darr))" "ul(color(white)(uarr darr))" "ul(color(white)(uarr darr)))#
#" "" "" "" "" "" "color(white)(/)2p#
#" "#
#ul(uarr darr)#
#color(white)(/)2s#
Since beryllium needs to form two identical
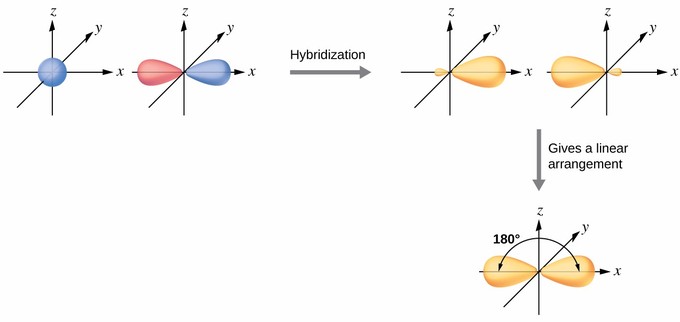
Pay close attention to the relative orbital energies now, which are shown below:
#" "" "" "" "" "" "underbrace(ul(color(white)(uarr darr))" "ul(color(white)(uarr darr)))#
#" "" "" "" "" "" "" "color(white)(./)2p#
#" "#
#ul(uarr color(white)(darr))" "ul(uarr color(white)(darr))#
#color(white)(/)sp" "" "sp#
#" "#
#ul"2s orbital was previously here in energy!"#
Due to this mixing,
- One previously pure
#2p# orbital from above is lowered in energy to form an#sp# orbital. - One previously pure
#2s# orbital rises in energy a bit to form an#sp# orbital. - The two electrons previously in the
#2s# atomic orbital of beryllium can now spread out amongst the two hybrid#sp# orbitals.
And this yielded two
They are identical orbitals, which must then make identical bonds.
Bond being made:
#overbrace("H")^(1s) -> larr overbrace("Be")^(sp) -> larr overbrace("H")^(1s)#
Bond made:
#" "" ""H"stackrel(1s-sp)stackrel("bond")stackrel(darr)(—)"Be"stackrel(sp-1s)stackrel("bond")stackrel(darr)(—)"H"#

